Introduction
Learning objectives: You will learn about interventional study design and its strengths and weaknesses.
Interventional studies are often performed in laboratories and clinical studies to establish beneficial effects of drugs or procedures. The present section introduces the readers to randomised controlled study design.
Read the resource text below.
Resource text
Intervention studies are considered to provide the most reliable evidence in epidemiological research. Intervention studies can generally be considered as either preventative or therapeutic [1].
Therapeutic trials are conducted among individuals with a particular disease to assess the effectiveness of an agent or procedure to diminish symptoms, prevent recurrence, or reduce mortality from the disease.
Preventative trials are conducted to evaluate whether an agent or procedure reduces the risk of developing a particular disease among individuals free from that disease at the beginning of the trial, for example, vaccine trials. Preventative trials may be conducted among individuals or among entire communities
Types of experimental interventions may include:
- - Therapeutic agents
- Prophylactic agents
- Diagnostic agents
- Surgical procedures
- Health service strategies
Characteristics of an intervention study
* A distinguishing characteristic of an intervention study is that the intervention (the preventative or therapeutic measure) being tested is allocated by the investigator to a group of two or more study subjects (individuals, households, communities).
* Subjects are followed prospectively to compare the intervention vs. the control (standard treatment, no treatment or placebo).
The main intervention study design is the randomised controlled trial (RCT).
Cross-over trials
A pre-post clinical trial/cross-over trial is one in which the subjects are first assigned to the treatment group and, after a brief interval for cessation of residual effect of the drug, are shifted into the placebo /alternative group. Thus, the subjects act as their own control at the end of the study. However, such studies are not feasible if there is mortality, or if the disease is easily cured by one of the interventions.
Randomised controlled trials
The randomised controlled trial is considered as the most rigorous method of determining whether a cause-effect relationship exists between an intervention and outcome [2]. The strength of the RCT lies in the process of randomisation that is unique to this type of epidemiological study design.
Generally, in a randomised controlled trial, study participants are randomly assigned to one of two groups: the experimental group receiving the intervention that is being tested and a comparison group (controls) which receives a conventional treatment or placebo. These groups are then followed prospectively to assess the effectiveness of the intervention compared with the standard or placebo treatment.
The random allocation of subjects is used to ensure that the intervention and control groups are similar in all respects (distribution of potential confounding factors) with the exception of the therapeutic or preventative measure being tested. The choice of comparison treatments may include an existing standard treatment or a placebo (a treatment which resembles the intervention treatment in all respects except that it contains no active ingredients).
Note that ethical constraints limit the choice of comparison treatments.
Figure 1. General outline of a two armed randomised controlled trial.
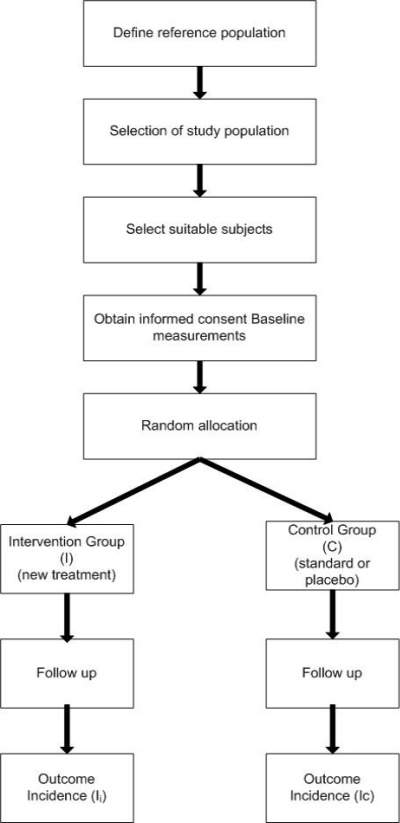
Basic outline of the design of a randomised controlled trial
1. Development of a comprehensive study protocol. The study protocol will include:
- - Aim and rationale of the trial
- Proposed methodology/data collection
- Definition of the hypothesis
- Ethical considerations
- Background/review of published literature
- Quality assurance and safety
- Treatment schedules, dosage, toxicity data etc.
2. Formulation of hypothesis.
3. Objectives of the trial.
4. Sample size calculations.
5. Define reference population.
6. Choice of a comparison treatment - placebo or current available best treatment.
7. Selection of intervention and control groups, including source, inclusion and exclusion criteria, and methods of recruitment.
8. Informed consent procedures.
9. Collection of baseline measurements, including all variables considered or known to affect the outcome(s) of interest.
10. Random allocation of study participants to treatment groups (standard or placebo vs. new).
11. Follow-up of all treatment groups, with assessment of outcomes continuously or intermittently.
12. Monitor compliance and losses to follow-up.
13. Interim analysis.
14. Analysis - comparison of treatment groups.
15. Interpretation (assess the strength of effect, alternative explanations such as sampling variation, bias).
16. Publication.
Randomisation
The aim of randomisation is to ensure that any observed differences between the treatment groups are due to differences in the treatment alone and not due to the effects of confounding (known or unknown) or bias. That is, that the groups are similar in all respects with the exception of the intervention under investigation.
Methods of random allocation are used to ensure that all study participants have the same chance of allocation to the treatment or control group, and that the likelihood of receiving an intervention is equal regardless of when the participant entered the study. Therefore, the probability of any participant receiving the intervention or the standard treatment/placebo is independent of any other participant being assigned that treatment.
The assignment of study subjects to each intervention is determined by formal chance process and cannot be predicted or influenced by the investigator or participant. In a well designed RCT, random allocation is determined in advance.
Methods of randomisation - allocation of subjects to intervention and control groups
1. Simple randomisation
For example, computer generated random number tables. Simple randomisation is rarely used.
2. Block randomisation
Block randomisation is a method used to ensure that the numbers of participants assigned to each group is equally distributed and is commonly used in smaller trials.
3. Stratified randomisation
Stratified randomisation is used to ensure that important baseline variables (potential confounding factors) are more evenly distributed between groups than chance alone may assure. However, there are a limited number of baseline variables that can be balanced by stratification because of the potential for small numbers of subjects within each stratum.
4. Minimized randomisation
This method may be used when the study is sufficiently small and simple randomisation will not result in balanced groups.
Note that deterministic methods of allocation such as by date of birth or alternate assignment to each group are not considered as random.
Advantages of randomisation
- Eliminates confounding - tends to create groups that are comparable for all factors that influence outcome, known, unknown or difficult to measure. Therefore, the only difference between the groups should be the intervention.
- Eliminates selection bias.
- Gives validity in statistical tests based on probability theory.
- Any baseline differences that exist between study groups are attributable to chance rather than bias. Though this should still be considered as a potential concern.
Disadvantages of randomisation
Does not guarantee comparable groups as differences in confounding variables may arise by chance.
Blinding in randomised controlled trials
Blinding is a process where the critical information on allocation of treatment is hidden either from the patients, or from observer or the evaluator in the study. The method of blinding in RCT is used to ensure that there are no differences in the way in which each group is assessed or managed, and therefore minimize bias. Bias may be introduced, for example, if the investigator is aware of which treatment a subject is receiving, as this may influence (intentionally or unintentionally) the way in which outcome data is measured or interpreted. Similarly, a subject's knowledge of treatment assignment may influence their response to a specific treatment.
Blinding also involves ensuring that the intervention and standard or placebo treatment appears the same.
Double blinding is when neither the investigator nor the study participant is aware of treatment assignments. However, this design is not always possible.
A single blind RCT is when the investigator but not the study participants know which treatment has been allocated.
Strengths of a randomised controlled trial
- A well designed randomised control trial provides the strongest evidence of any epidemiological study design that a given intervention has a postulated effectiveness and is safe.
- A RCT provides the best type of epidemiological study from which to draw conclusions on causality.
- Randomisation provides a powerful tool for controlling for confounding, even by factors that may be unknown or difficult to measure. Therefore, if well designed and conducted, a RCT minimizes the possibility that any observed association is due to confounding.
- Clear temporal sequence - exposure clearly precedes outcome.
- Provides a strong basis for statistical inference.
- Enables blinding and therefore minimizes bias.
- Can measure disease incidence and multiple outcomes.
Weaknesses of a randomised controlled trial
- Ethical constraints - for example, it is not always possible or ethical to manipulate exposure at random.
- Expensive and time consuming.
- Requires complex design and analysis if unit of allocation is not the individual.
- Inefficient for rare diseases or diseases with a delayed outcome.
- Generalisability - subjects in a RCT may be more willing to comply with the treatment regimen and therefore may not be representative of all individuals who might be given the treatment.
References
1. Hennekens CH, Buring JE. Epidemiology in Medicine, Lippincott Williams & Wilkins, 1987.
2. Kendall JM. Designing a research project: Randomised Controlled trials and their principles, Emerg Med J. 2003, March;20(2)164-168.
3. Hollis S, Campbell F, What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ 1999; 319;670-74.
Further Resources
1. Pocock SJ. Clinical Trials: A practical approach, Chichester, Wiley, 1984.
2. Sibbald B, Roland M, Understanding controlled trials: Why are randomised controlled trials important?, BMJ 1998, 316:201.
3. Altman DG, Randomisation. BMJ 1991;302;1481-2.
