Introduction
Learning objectives:You will be able to understand a cohort design, understand the differences from a case-control design, calculate the basic measures (relative risk, attributable risk etc), and appreciate its strengths and weaknesses.
Cohort studies are a form of longitudinal study design that flows from the exposure to outcome. This section outlines the challenges in designing such studies, their analysis, and interpretation of outcomes.
This section also covers:
1. Issues in the design of cohort studies
2. Potential bias in cohort studies
3. Analysis of cohort studies
4. Strengths and weaknesses of cohort studies
Read the resource text below.
Resource text
In a cohort study, a group of individuals exposed to a putative risk factor and a group who are unexposed to the risk factor are followed over time (often years) to determine the occurrence of disease. The incidence of disease in the exposed group is compared with the incidence of disease in the unexposed group. The relative risk (incidence risk or incidence rate) is used to assess whether the exposure and disease are causally linked. Cohort studies may be prospective or retrospective. A prospective cohort study is also called a concurrent cohort study, where the subjects have been followed up for a period and the outcomes of interest are recorded.
In a retrospective cohort study both the exposure and outcome have already occurred at the outset of the study. While this type of cohort study is less time consuming and costly than a prospective cohort study, it is more susceptible to the effects of bias. For example, the exposure may have occurred some years previously and adequate reliable data on exposure may be unavailable or incomplete. In addition, information on confounding variables may be unavailable, inadequate or difficult to collect.
1. Issues in the design of cohort studies
Selection of study groups
The aim of a cohort study is to select study participants who are identical with the exception of their exposure status. All study participants must be free of the outcome under investigation and have the potential to develop the outcome under investigation.
Measuring exposure
Levels of exposure (e.g. packs of cigarettes smoked per year) are measured for each individual at baseline at the beginning of the study and assessed at intervals during the period of follow-up. When several exposures are being considered simultaneously, the non-exposed group should comprise all those with none of the risk factors under investigation.
A particular problem occurring in cohort studies is whether individuals in the control group are truly unexposed. For example, study participants may start smoking or they may fail to correctly recall past exposure. Similarly, those in the exposed group may change their behaviour in relation to the exposure such as diet, smoking or alcohol consumption.
Exposure data may be obtained from a number of sources including medical or employment records, standardized questionnaires, interviews and by physical examination.
Measuring outcome
Outcome measures may be obtained from various sources, including routine surveillance of cancer registry data, death certificates, medical records or directly from the participant. Note that the method used to ascertain outcome must be identical for both exposed and unexposed groups.
Methods of follow-up
The follow-up of study participants in a cohort study is a major challenge. A great deal of cost and time is required to ensure follow-up of cohort members and to update measures of exposures and confounders, in addition to monitoring participants' health outcomes. The failure to collect outcome data for all members of the cohort will affect the validity of study results.
2. Potential sources of bias in cohort studies
A major source of potential bias in cohort studies is due to losses to follow-up. Cohort members may die, migrate, change jobs or refuse to continue to participate in the study. In addition, losses to follow-up may be related to the exposure, outcome or both. For example, individuals who develop the outcome may be less likely to continue to participate in the study. The degree to which losses to follow-up are correlated with exposure and outcome will lead to serious bias in the measures of effect of exposure and outcome [1].
A major source of potential bias in cohort studies arises from the degree of accuracy with which subjects have been classified with respect to their exposure or disease status. Differential misclassification can lead to an over or underestimate of the effect between exposure and outcome [1].
Selection bias in cohort studies
Selection bias is a potential problem in case-control studies. Selection bias may be introduced when the completeness of follow-up or case ascertainment differs between exposure categories. This can be minimized by ensuring that a high level of follow-up is maintained among all study groups
The healthy worker effect
The healthy worker effect is another potential form of selection bias in cohort studies, particularly affecting occupational studies. In an occupational cohort study where disease rates among individuals from a particular occupational group are compared with an external standard population, bias may be introduced if membership of the exposed cohort is partly dependent upon health (which may be related to the presence or absence of the health outcome under investigation).
Individuals who are employed, for example, are generally healthy by nature of their ability to work. Therefore, mortality or morbidity rates in the occupation group cohort may be initially lower than in the population as a whole, which includes individuals who are too ill to work.
In order to minimize the potential for this form of bias, a comparison group may be selected from a group of workers with different jobs performed at different locations within a single facility [1]. For example, a group of non-exposed office workers. Alternatively, the comparison group may be selected from an external population of employed individuals.
3. Analysis of cohort studies
Analysis of a cohort study uses either the risk or the rate ratio of disease in the exposed cohort compared with the rate or risk in the unexposed cohort.
Note that the risk ratio uses as a denominator the entire group recruited at the start of the study, while the rate ratio uses as a denominator the person years, which takes account of losses to follow-up.
Table 1. Calculation of the rate ratio from a hypothetical cohort study of smoking and cancer of the pancreas followed for 1 year.
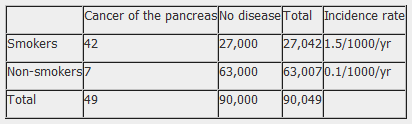
From the data in table 1, taken from a hypothetical cohort study to investigate the association between smoking and cancer of the pancreas, the relative and attributable risk can be calculated as follows:
Rate Ratio = Incidence rate in exposed group (r1)
Incidence rate in unexposed group (r0)
RR = 1.5/0.1 = 15
The relative risk of 15 indicates that the risk of cancer of the pancreas is 15 times higher among smokers than non-smokers.
4. Strengths and weaknesses of cohort studies
Strengths
Weaknesses
References
1. Hennekens CH, Buring JE. Epidemiology in Medicine, Lippincott Williams & Wilkins, 1987.
- Multiple outcomes can be measured for any one exposure.
- Can look at multiple exposures.
- Exposure is measured before the onset of disease (in prospective cohort studies).
- Good for measuring rare exposures, for example among different occupations.
- Demonstrate direction of causality.
- Can measure incidence and prevalence.
- Costly and time consuming.
- Prone to bias due to loss to follow-up.
- Prone to confounding.
- Participants may move between one exposure category.
- Knowledge of exposure status may bias classification of the outcome.
- Being in the study may alter participant's behaviour.
- Poor choice for the study of a rare disease.
- Classification of individuals (exposure or outcome status) can be affected by changes in diagnostic procedures.
